CGM use in healthy individuals - Hype, hope or necessity?
Continuous glucose monitors have been the fastest growing technologies for years. Whilst targeted towards individuals living with Type I Diabetes, their use has extended to Type II Diabetics on insulin to those interested in improving or tracking their metabolic health. This article dives into the current state of the science, market and trends.
.
Written by Mariette Abrahams PhD MBA
The scale of the problem of Metabolic health
Metabolic health is a growing concern around the globe. Recent statistics indicate that there are approximately 537 million adults aged between 20 – 70 years diagnosed with Diabetes (IDF). This represents around 12% of the global population. This number is projected to grow to 700 million by 2045. To put this in perspective, by 2045 the world population would have grown by 20%, but the incidence of Diabetes would have increased by 46%
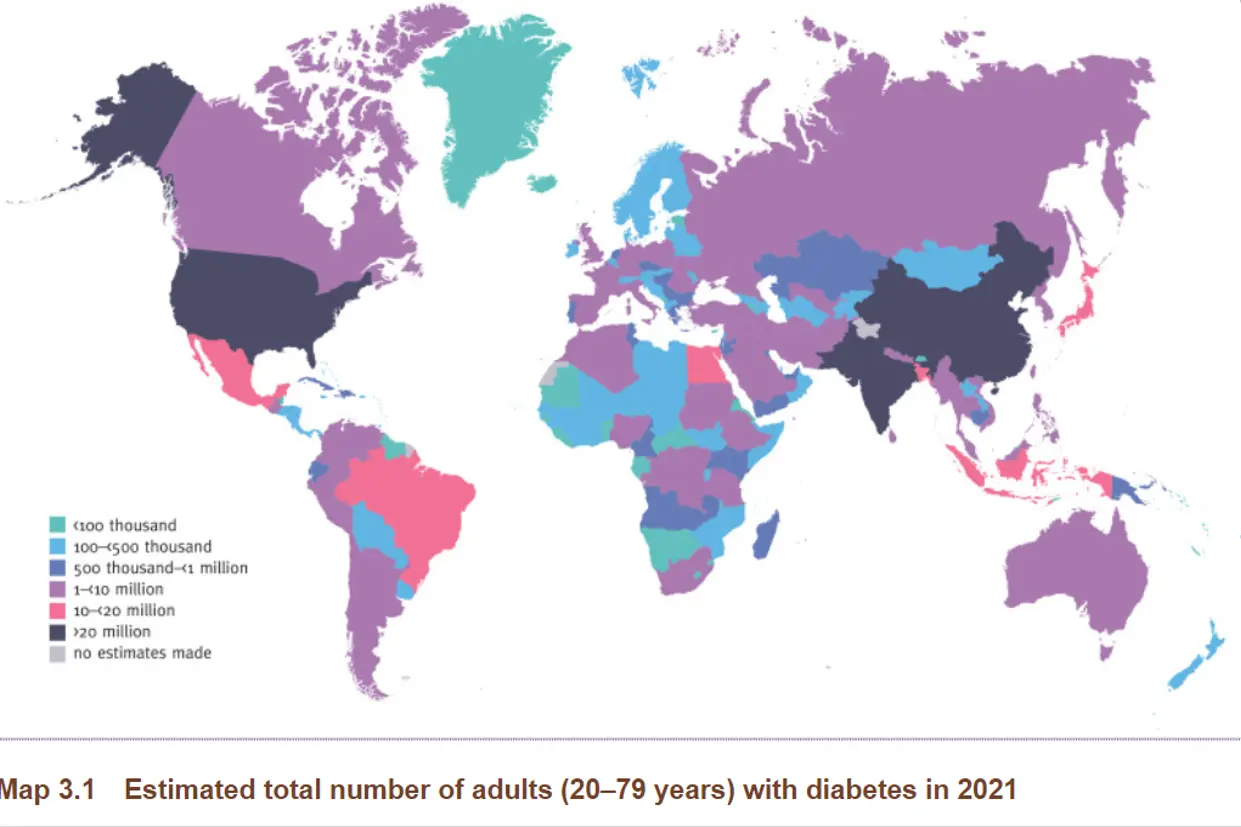
Countries leading on the Diabetes charts include the Unites States, China and India. This rapid rise can be attributed to a growing aging population, increased disposable income in emerging markets as well as alarmingly high rates of obesity. Obesity alone increases risk of Diabetes 3 times.
In the US, 1 in 10 American live with Diabetes. This translates into around 37 million adults. Of these 90-95% (nearly 29 million) Have Type II Diabetes according to the CDC. These numbers account for adults having a received a diagnosis which equals to 1.2 million each year. However, most adults are unaware that they have Prediabetes which is estimated to be in the region of 96 million of which 70% will go on to develop Diabetes. In fact a recent study conducted in the US estimated that less than 7% of the US adult population is metabolically healthy (O' Hearn 2022)
The annual cost of diabetes in the USA is $327b. Individuals with diagnosed diabetes incur $16,752 in health care expenses per year, which is 2.3 times greater than individuals without diabetes. In short, metabolic disorders is a big problem with big money (and global health) at stake.
Current Management of Metabolic health disorders
Gold standard interventions to manage metabolic disorders such as Type II Diabetes, Gestational Diabetes and Metabolic syndrome include diet, lifestyle, medications and at the extreme end, surgery.
Three major studies in the USA, Finland, and China have shown that lifestyle interventions, including dietary weight loss and physical activity, can prevent diabetes progression in high-risk individuals such as those living with PreDiabetes by 51–58% from 2.9 - 6 years after the original intervention, with continued protection of 27–43% for up to 20 years. This means that diet and lifestyle play a huge role in the management of metabolic disorders. Programs such as the Diabetes Prevention programs have been implemented for years which is a structured program of education and monitoring that help individuals that are at risk or newly diagnose to manage their condition.
Diabetes prevention programs, while cost effective, are resource-intensive and require contact with health care professionals/systems that may not be accessible to all, with reported participation rates as low as 2.6% due to lack of physician referrals and socioeconomic barriers.
During the first 4 years of implementation of the National DPP effort, only 39% of participants were retained at 44 weeks, and only 35.5% achieved the 5% weight loss goal (Venkataramani et al 2019; Cannon et al 2020).
It is common practice in the clinical setting, that individuals living with Type II Diabetes do not have to monitor their blood sugar levels (using finger pricks) as it has been found by research to not impact HbA1C levels (ADA). This recommendation or practice is followed despite the fact that adherence to Diabetes medications is only 40-50%, thereby greatly contributing to the advancement of the disease (Kleinsinger 2018), and only half of Diabetics reach glycemic and blood pressure control. (Fang et al 2021). Having an insight into daily blood sugar levels using new technologies can be a way of understanding how daily habits impact blood sugar levels in real-time. This opens the door to leveraging new technologies to provide Digital health.
Managing Blood sugar with Digital health
One of the fastest growing health technologies have been continuous glucose monitors or CGM’s. These little sensors are placed on the back of the upper arm and last up to 10-15 days. The microneedle that is inserted into the skin is coated with an enzyme glucose oxidase that reacts to the glucose concentration in the interstitial fluid and transmits a signal via bluetooth to a device such as a smartwatch or smartphone every 5 – 15 minutes.
The blood glucose picked up by the sensor fluctuates with the changes in glucose % throughout the day as the body responds to diet, stress, sleep, physical activity. CGM’s open up the opportunity for self and remote care. Blood glucose reading via a sensor lags by about 5 - 20 minutes than blood glucose obtained via finger prick.
By 2045 the world population would have grown by 20%, but the incidence of Diabetes would have increased by 46% - IDF
CGM Market overview
The CGM market is consolidated with 3 main players. Abbott who manufacture the Freestyle Libre sensor is the market leader holding just over 50% of the market share. Dexcom leads next around 39% market share and smaller players such as Medtronic 8%. The CGM market is growing rapidly and was valued at $5.33 billion in 2022 and is estimated to surpass $12.39 billion by 2030 with a CAGR of 10.6% (Globalnewswire).
Factors driving uptake of CGM’s
- Increased awareness and knowledge among prescribers (Peek et al)
- Increased support by CGM companies
- Increased accessibility (Kompala et al 2019)
- Improved CGM technology
- Ancillary services such as pharmacy that work with prescribers to adjust medication and find providers and provide education
- Most recently both Abbott and Dexcom have had their over the counter CGM sensor approved by the FDA. This increased accessibility will no doubt drive further adoption. The Abbott Lingo has been available in the UK for some time.
What are the benefits of wearing CGM in Diabetes:
- Better experience - No finger pricking
- 24/7 feedback
- Convenience of not having to carry around equipment
- Cost saving in long term management (Laiteeranpong et al 2018)
- Better Diabetes management
- Lower HbA1C
- Improved quality of life
Based on a recent report, while there are around 2.4 million CGM Diabetic users, of these 70% are Type I Diabetics and only 3-4% are Type II. (Seagrove Partners 2021)
CGM’s as biofeedback in healthy individuals for Personalized nutrition
Interest in metabolic health has been steadily growing owing to prolific social media influencers such as Dr Peter Attia and the Glucose goddess promoting the importance of stable blood sugar levels for optimal health over the last few years. Despite a lack of strong scientific evidence for the use of CGM’s in healthy individuals, the market continues to grow, evidenced by a burgeoning startup ecosystem who offer solutions targeting blood sugar levels for a range of applications.
The premise of the argument for “optimal” blood sugar levels or monitoring daily blood sugar in healthy individuals relies in recent evidence that suggest that:
- Individuals do not respond to the same foods in the same way even identical twins (Predict)
- Traditional gold-standard methods such as HbA1C do not adequately identify metabolic dysregulation in individuals who may present with normal HbA1C levels
- Individual glucose variation is impacted by sleep, stress, time of day, menstrual cycle, life stage which is not reflected in HbA1C or oral glucose tolerance tests
- Maintaining tight blood glucose control can increase lifespan and healthspan.
The difference between blood targets and levels Diabetics and Healthy individuals
Currently there is no established agreement on the blood sugar level range for healthy individuals, as traditionally these have been based on the ones as defined for Diabetes care and management. Glucose variation in response to event should be below 20% in healthy individuals.
Based on two recent papers, Shah et al (2019) found that healthy individuals spent over 96% TIR (Time in range) of 70 – 140mg/dL.
Another recently published paper with a cohort of 12 540 individuals composed of 23% Female, physically active with a normal BMI (23), spent 85% in range (Skroce et al 2024). There was a slight difference between males and females in that females spent less time above range (TAR) referring to blood sugar levels >140mg/dL and more time below range (TBR) defined as blood sugar <70mg/dL.
Despite incredible interest in glucose variations or blood glucose spikes, the most important indicator of optimal blood sugar management the Post prandial (post meal) glucose response, which is the blood sugar level up to 2 hours after eating a meal.
Overall, the data suggest that the current range for blood sugar control is appropriate in healthy individuals, but that these may need to be personalized based on the individual profile. This personalization can be offered for different health goals such as sports performance, cognitive health, gut health, menopause, sleep, Longevity, PCOS, migraines to name a few.

Source: Shah et al 2019
Current dietary approaches to lower blood sugar levels
The old way of carbohydrate counting and extreme diets are long gone. The ADA no longer recommend specific diets to prevent or manage Diabetes, but rather promote the consumption of a dietary pattern that is higher in fiber, lower in saturated fats, higher in protein and lower in total carbohydrate content (ADA). This means that dietary patterns such as Low Glycemic index (GI/GL) have made a comeback, and dietary patterns such as low carbohydrate, Mediterranean and Flexitarian or commonly recommended.
Glycemic index (GI) and glycemic load are tools to measure the impact of carbohydrate on blood sugar levels. Glycemic index is based on eating 50g of a specific food while glycemic load is based on a standard serving size of a specific food. The glycemic index of a food is calculated out of a score of 100, with pure sugar receiving a score of 100. The lower the GI of a food the lower the impact of food on blood sugar levels. However, this is only part of the story as it does not indicate how the food will actually affect sugar levels when eaten. Glycemic load which refers to the actual amount of carbohydrate consumed as part of the portion is also expressed as a number.
This also includes approaches indirectly by targeting the microbiome to improve glycemic control (Mendes- Soares et al 2021). The ultimate goal is to get individuals to adhere to a dietary plan that can lead to a significant weight loss of over 5 – 7 % as obesity is the leading contributor to Type II Diabetes.
Companies offering solutions in the personalized nutrition market combine the use of a CGM with personalized dietary advice, recommendations for physical activity, coaching and education to stay in the optimal range for blood sugar.

Difference between CGM’s used in Diabetes and for Healthy individuals
CGM sensors currently available on the market come equipped with a simple app that can aggregate, summarize and visually display readings, patterns and trends. There is a slight overlap between the sensors used in the management of Diabetes and those for healthy individuals. In general they both read the normoglycemic range of 70mg/dL - 140mg/dL, however at lower or above the range, sensors used in Diabetes will send of alarms notifications. This is not the case in the sensors used for lifestyle products. For instance the Abbott sense which was the sensor used by Supersapiens (now closed) did not read below 70mg/dL or above 140mg/dL. One would just receive a standard note on the chart that the results was too low or too to read.
Current Personalized nutrition CGM companies
At Qina we track the entire personalized nutrition industry by segment, technology and more. Below are 4 companies that offer Personalized nutrition solutions that includes wearing a CGM for at least 10 – 14 days.
Levels – Is a US based company launched in 2019. They offer a program which includes access to an app, creation of a metabolic score based on your blood sugar responses as well as access to educational resources and additional behaviour change support techniques for habit creation. Generally more targeted towards metabolic health, holistic health, Levels is one of the earliest and biggest players.
Zoe- Is a UK based start-up that has published the PREDICT trials on which the algorithm is based. Their solution includes wearing a CGM for 10 days, doing a stool test as well as a blood-draw using a state-of the art device to measure nutritional biomarkers after a challenge test (eating special cookies). The program includes access to educational resources, nudges, access to nutrition coaches via chat, a metabolic score, recipes, AI food logging and follow – up tests. Zoe targets individuals who are interested in improving their holistic and gut health.
January AI – Is a US based startup that has also published a lot of their research. Their solution includes wearing a CGM for 10 days whilst logging food, mood and physical activity. The solution then provides a metabolic score and provides personalized dietary and lifestyle advice using a chatbot. January AI targets individuals who are interested in understanding their metabolic health, PreDiabetics and Diabetics.
Signos – Is a US based startup that targets weight loss. The solution includes wearing a CGM and getting access to nutrition and behavioral coaching. The solutions provides educational resources, access to recipes and instructional video’s.
Healthy individuals spent over 96% of their time in range - Shah et al 2019.
Current state of the science of CGM use in Personalized nutrition
The body of evidence is still thin for the use of CGM’s in healthy individuals, however large digital trials are ongoing with some of the players such as Levels, HelloInside and Nutrisense who are looking to validate their approach.
A number of small studies already published indicate the potential for integrating CGM’s into a digital and personalized health approach.
In one small study conducted in obese individuals, CGM use led to increased motivation to exercise N=19) (Liao 2020)
In another survey based study conducted in Type II Diabetics on and without insulin reported a 87% increased motivation to make healthier food choices and avoid foods that cause significant spikes in sensor glucose levels (Erhardt et al 2019)
In a larger study (N = 665) conducted in the US among a mixed cohort of healthy, Pre and Type II Diabetics without insulin, they reported a 20% improvement in TIR after 10 days of wearing a CGM and receiving personalized feedback (Zahedani et al 2021)
Another small study (N=40) demonstrated enhanced weight loss and improved lipid profile on a low GI/GL diet in obese young adults over 8 weeks (Chikima et al 2022)
Wyatt et al 2022 demonstrated that glucose dips (variability) can predict hunger and energy intake 2-3hrs post prandially in the PREDICT 1 cohort (N = 1070).
Jospe et al (2020) demonstrated that wearing a CGM could enhance adherence to protocol to eat according to hunger cues
In another recent study conducted in Germany among overweight mostly female migraine sufferers (N=49), the intervention group received a CGM, dietary challenges, an app as well as nutrition expert coaching to nudge participants towards consuming a low GI dietary pattern. The researchers found that the Digital therapeutic. Resulted in a reduction of 2.65 migraine days. They also reported that migraine patients had slightly increased mean glucose values compared to healthy controls, but that they drop into a glucose range that is below one’s individual standard range just before a migraine attack (Lelleck et al 2022)
The UK based Zoe company also recently published the results of their Zoe methods trial. The researchers reported that their approach which includes wearing a CGM, a challenge test meal, at home blood and stool tests as well as personalized diet scores and education resulted in significantly lower Triglyceride levels, as well as a reduction in weight and waist circumference in highly adherent individuals.
Our perspective on the Zoe methods study will be published in another post....
Most recently, a study conducted in the US as part of the Diabetes prevention program, found that adding a CGM and education session at the start of the 18 week lifestyle program increased awareness among participants and was also acceptable (Richardson et al 2024)
This is in contrast to a paper published by the NIH which recommends that CGM's should not be used to determine glucose response to foods, as the glucose variability between identical meals (that contain more than one component) are as wide as two entirely different meals.(Hengist 2024)
Can wearing a CGM lead to meaningful and sustainable behavior change?
Several small proof of concept studies have been published, showing that individuals with T2D using CGM chose lower glycemic index foods, increased physical activity, decreased caloric intake, lost weight , and demonstrated decreased postprandial glucose levels.
In a recent retrospective study of 567 participants which consisted of Healthy, PreDiabetic and Type II Diabetics (Zahedani 2023)
Among individuals with prediabetes, there is only one published study addressing the role of CGM in promoting behavior change, and, while it showed greater dietary self efficacy, neither weight nor glycemic measures were reported (Mendes-Soares 2019)
Yoo et al. randomized patients with poorly controlled T2D (baseline HbA1c of 9%) who were treated with insulin (60%) or non-insulin therapies (40%), to real-time CGM vs. self monitoring blood glucose (SMBG) and demonstrated significantly greater reduction in HbA1c (0.50%, p = 0.004) at 12 weeks in the CGM group, along with reduction in total daily calorie intake, greater weight loss, and increased physical activity.
In a qualitative study conducted by Johnston (2022) Self-care was a determinant of quality of life. Participants reported that the clearest benefit from CGM use is greater awareness of glucose levels, things that affect those levels, and how increased awareness provides users greater ability to care for their health. The researchers suggest that CGM use, and the increased awareness of glucose levels it provides, encouraged respondents to adjust their diets and physical activity. More specifically participants reported that they were more consistent about adjusting their eating based on their sugars levels and that they were overall more consistent in doing things to take good care of their health. This indicates that participants demonstrated a higher level of self-efficacy.
A recent scoping review conducted by Richardson et al (2023) found that biofeedback which includes the provision of feedback based on a biomarker, is increasingly used a behavior change technique.
In a recent exploratory secondary analysis conducted by Jospe et al (2024), researchers found an improvement in glycemic variability in women at risk of postmenopausal breast cancer consuming a low GI diet. And most recently, a systematic review demonstrated modest but favorable effects of CGM based feedback in both individuals living with and without Diabetes. (Richardson et al 2025)
In short, while CGM’s or data thereof is increasingly used in new solutions to provide biofeedback, there efficacy of this approach still remains unknown.

Current challenges and gaps
CGM’s have been one the fastest growing medical technologies, however numerous challenges still exist which include:
- Cost – CGM’s can cost anywhere from $100 - $300 per month which can be inaccessible to many individuals who would benefit the most. In one exploratory study, researchers found that 1 in 10 (10%) respondents discontinued use of the devices due to cost. Similarly, ten percent of the survey respondents reported discontinuing use due to high cost and nearly 40% reported having to pay for the full cost of the device out of pocket, because it was not covered at all by their insurance provider (Sears et al 2022.)
- Reimbursement – for sensor use is not accessible to all. At present around 70% of Type I Diabetics use a CGM and they would have sensors reimbursed by insurance. However not all insurers reimburse sensor use in individuals with Type II Diabetes. This means that individuals either need to pay out of pocket or go without. Access for PreDiabetes, Gestational Diabetes and those looking to prevent Diabetes is down to the prescribing physician who may be open to prescribe but cannot guarantee that the sensor would be reimbursed.
- Lack of knowledge – historically mostly as Endocrinologist prescribed insulin regimens (Warman et al 2022) which means there is a bottleneck for those Type II diabetics who are not on insulin.
- Equity – CGM's are not distributed or available equitable with discrepancies reported for age, gender and ethnicity. According to the NIH individuals earning less than $30 000 per year are three times more likely to live with Diabetes in comparison to those earning over $80 000 per year (ADA, 2022). Interestingly the ADA reports that uninsured individuals with Diabetes are prescribed 52% fewer medications and are less likely to get access to a CGM are people of color, those on low income and individuals living in states with high rates of Diabetes (ADA, 2022)
- Disordered eating having 24/7 access to glucose data in your hand combined with an urge to “flatten the curve” can lead to obsessive behavior with regard to food and physical activity. This can be true for individuals who may be at risk of eating disorders, however an exact estimate for how often this happens is not clear and this could be said for all digital health or nutrition programs.
- Performance CGM's are not failure proof and can often stop measuring before their due date of 12-14 days. Poor performance has been cited as one of the most common reasons individuals stop using a CGM (Medtech dive)
- Lack of insight into healthfulness of a meal - A meal or product that does not lead to a blood sugar spike is not necessarily reflective of its healthfulness. For instance a bowl of ice cream may lead to a lower spike than a piece of fruit because of it's composition. Higher content of protein and fat can lead to lower spikes but not always more nutrition.
- Lag time can be misleading - Most of the research has been focused on the 2 hour post-prandial period. This means, blood sugar levels should drop back to the "normal range" within a 2 hour period, this is a normal response. However, in practice this can lead to confusion. As a CGM measures interstitial glucose measuring the glucose concentration between cells (not actual blood glucose), there can be a lag time of 5-20 minutes. This means that measuring glucose using a CGM and fingerprick at the same time will not lead to the same results.
Accuracy of CGM’s in healthy individuals
Sensor glucose levels measured by CGM are considered to be within close proximity of the venous blood glucose levels, but there are several factors influencing the discrepancy between them. Studies have shown that accuracy of CGM can range from 7.8 % to 12.2% (Ferch 2021,Soni 2022, Moser 2022, Shridhara 2022). This means that at any given time as measured in clinical setting, compared to venous blood sample, the values reported by CGM were different by 12.2% on average. Accuracy of blood glucose meters using capillary blood can range between 5% and 8% Accuracy of CGM’s are a hot topic as they can vary and sensor failures can be common.
Accuracy of sensors are measured by MARD score. A MARD score below 10 indicates good to high accuracy, whilst a MARD score over 20 indicates poor accuracy. Over the past 20 years, MARD scores of the most commonly used sensors have come down from 25% in 1990 to around 7% (Bailey et al 2021) which indicates a high level of accuracy, but this is not always reflected in the real-world experiences of wearers who experience high variability.
Factors that can affect accurate readings:
- Sleep
- Stress
- Medication
- Incorrect placement of the sensor
- Temperature
Market developments and future outlook
CGM manufacturers have quickly realized the growing interest of CGM in the growing metabolic health market. While companies such as Abbott and Dexcom still largely focus on the Type II Diabetes population, the fact that a sensor needs to be prescribed is a barrier to widespread adoption. Both Abbott and Dexcom have announced the launch of sensors that will be available without a prescription for individuals who are not on insulin. The Abbott Lingo is already available in the UK. As the two market leaders enjoy over 80% of the metabolic health market, we suspect that the low price, easy access and longer lifespan of the sensor will drive new adoption across the globe.
Weight watchers also recently announced partnership with Abbott on a new study which aims to investigate whether wearing a CGM leads to superior results
Dexcom partnered with Garmin which means access to the fitness and active lifestyle consumer group
Huwaei last year announced the launch of their glucose sensing watch whilst Apple has been developing their for a while now but not yet launched. In essence, we will see more non-invasive solutions that aim to measure glucose coming onto the market.
The Qina take – to wear or not to wear a CGM?
Whether healthy individuals should wear a CGM is a hot topic among academics, practitioners, industry and consumers. In our view, healthy individuals who have established healthy habits and lifestyle will get little value from wearing a CGM especially in the short term. Curiosity will prevail in the end as CGM’s become widely visible (more people wearing them) and available. Individuals who have specific goals or concerns that are not met by traditional means is an area of opportunity as the evidence is building. The biggest concern is the lack of unequal access especially for at-risk groups who would benefit the most. Without comprehensive support and education, just wearing a CGM is unlikely to lead to lasting behavior change.
We should also not put all our eggs in the “glucose” basket as the levels are a results of a multitude of environmental factors such as sleep, stress, time of the cycle, time of day. This means that just cutting out entire food groups can lead to unachievable goals. In addition, it is one metabolite, whereas having access to other biomarkers could provide more quality insights.
Whether CGM’s can be used as an educational or motivational tool is still under investigation, but our initial view is that wearing a CGM could lead to behavior change in motivated individuals.
One area that is not discussed at all is the amount of medical waste that is generated from wearing sensors, considering that they are single-use, medical grade and not recyclable. Manufacturers should really find a solution considering that 1 sensor only lasts up to 15 days. With the global Diabetes population to grow to 700 million by 2045, that will mean an awful lot of CGM waste.
Conclusion
CGM’s have revolutionized treatment for Type I Diabetes patients and have slowly been adopted by individuals without Diabetes. The benefits of wearing a CGM are multifold, yet access is currently unequally distributed, with the ones that would benefit the most not benefiting from this technology. The competitive marketplace is active around the globe with many having positioning around holistic health, weight loss and performance. Individuals living with Type II Diabetes and the 29 million individuals living with PreDiabetes would benefit the most from wearing a CGM, yet adoption is only around 3-4%. With low adoption and the new sensors yet to hit the market this year, we expect that we are still at the early adopter stage and that we are yet to see the full scale impact of this next wave of precision health.
This is a write- up of a presentation recently delivered by Qina’s CEO & Founder Mariette Abrahams PhD MBA for the Bell institute of health. To see a full list of the 40 companies offering CGM solutions in our Qina engine, the first comprehensive database of the competitive Personalized nutrition landscape, learn more about our Explore service here.
References
Sharon M. Donovan, Mariette Abrahams, Joshua C. Anthony, Robert Bergia, Gil Blander, Tristin D. Brisbois, Anna-Sigrid Keck, Edwin G. Moore, Timothy A. Morck, Kristin M. Nieman, Jose M. Ordovas, Alison Steiber, Barbara L. Winters, Thuyvan Wu,Perspective: Challenges for Personalized Nutrition in the Current U.S. Regulatory Framework and Future Opportunities, Advances in Nutrition, 2025,100382, ISSN 2161-8313,
Mariette Abrahams & Matusheski N.V.(2020) Personalised nutrition technologies: a new paradigm for dietetic practice and training in a digital transformation era. J Hum Nutr Diet. 33, 295–298
Mariette Abrahams, Bryant E, Frewer L, Stewart-Knox B (2019) Personalised nutrition technologies: A cross-national survey of Registered Dietitians. Public Health Genomics DOI: 10.1159/000502915.
Stewart-Knox B, Gibney E, Mariette Abrahams , Rankin A, Bryant E, Oliveira BMPM, Poínhos R (2019) Personalised Nutrition: Making it Happen. In C. Galanakis (ed) Trends in Personalised Nutrition. London: Elsevier. https://shop.elsevier.com/books/trends-in-personalized-nutrition/galanakis/978-0-12-816403-7
Center for Disease Control and Prevention. Type 2 Diabetes 2021. Available from: https://www.cdc.gov/diabetes/basics/type2.html. Accessed 26 Sept 2022 [
Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in US adults, 1999–2018. N Engl J Med. 2021;384(23):2219–2228. doi: 10.1056/NEJMsa2032271
Beck RW, Riddlesworth TD, Ruedy K, Ahmann A, Haller S, Kruger D, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167(6):365–374. doi: 10.7326/M16-2855
Kompala T, Neinstein A. A new era: increasing continuous glucose monitoring use in type 2 diabetes. Am J Manag Care. 2019;25(4 Spec No.):Sp123–sp6.
Peek ME, Thomas CC. Broadening access to continuous glucose monitoring for patients with type 2 diabetes. JAMA. 2021;325(22):2255–2257. doi: 10.1001/jama.2021.6208.
Bergenstal RM, Layne JE, Zisser H, Gabbay RA, Barleen NA, Lee AA, et al. Remote application and use of real-time continuous glucose monitoring by adults with type 2 diabetes in a virtual diabetes clinic. Diabetes Technol Ther. 2021;23(2):128–132. doi: 10.1089/dia.2020.0396
Kompala T, Neinstein A. A new era: increasing continuous glucose monitoring use in type 2 diabetes. Am J Manag Care. 2019;25(4 Spec No.):Sp123–sp6
Kravarusic J, Aleppo G. Diabetes technology use in adults with type 1 and type 2 diabetes. Endocrinol Metab Clin North Am. 2020;49(1):37–55. doi: 10.1016/j.ecl.2019.10.006
Warman M, Filippi M, Manning B, Oser T, Nease D, Hall T, et al. Continuous glucose monitoring for primary care patients with diabetes: barriers, facilitators, & resources to support access. Ann Fam Med. 2022;20(Supplement 1):2689. doi: 10.1370/afm.20.s1.2689
Laiteerapong N, Cooper JM, Skandari MR, Clarke PM, Winn AN, Naylor RN, et al. Individualized glycemic control for U.S. adults with type 2 diabetes: a cost-effectiveness analysis. Ann Intern Med. 2018;168(3):170–8. doi: 10.7326/M17-0537
Eiland L, Thangavelu T, Drincic A. Has technology improved diabetes management in relation to age, gender, and ethnicity? Curr Diab Rep. 2019;19(11):111. doi: 10.1007/s11892-019-1231-5.
Mahajan S, Lu Y, Spatz ES, Nasir K, Krumholz HM. Trends and predictors of use of digital health technology in the United States. Am J Med. 2021;134(1):129–134. doi: 10.1016/j.amjmed.2020.06.033.
Mayberry eta l2023 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10228889/#CR4
Centers for Disease Control and Prevention. National Diabetes Statistics Report Website. https://www.cdc.gov/diabetes/data/statistics-report/index.html (2022).
Tabák, A. G., Herder, C., Rathmann, W., Brunner, E. J. & Kivimäki, M. Prediabetes: a high-risk state for diabetes development. Lancet 379, 2279–2290 (2012).
Nathan, D. M. et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 30, 753–759 (2007).
American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care 41, 917–928 (2018).
Knowler, W. C. et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 346, 393–403 (2002).
Li, G. et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 371, 1783–1789 (2008).
Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program outcomes study. Lancet Diabetes Endocrinol. 3, 866–875 (2015).
Lindström, J. et al. Improved lifestyle and decreased diabetes risk over 13 years: long-term follow-up of the randomised finnish Diabetes Prevention Study (DPS). Diabetologia 56, 284–293 (2013).
Li, G. et al. Effects of insulin resistance and insulin secretion on the efficacy of interventions to retard development of type 2 diabetes mellitus: the DA Qing IGT and diabetes study. Diabetes Res. Clin. Pract. 58, 193–200 (2002)
Venkataramani, M., Pollack, C. E., Yeh, H. C. & Maruthur, N. M. Prevalence and correlates of diabetes prevention program referral and participation. Am. J. Prev. Med 56, 452–457 (2019).
Cannon, M. J. et al. Retention among participants in the National Diabetes Prevention Program lifestyle change program, 2012-2017. Diabetes Care 43, 2042–2049 (2020)
Iqbal, S. M. A. et al. Advances in healthcare wearable devices. Npj Flex. Electron 5, 9 (2021)
Cox, D. J. et al. Continuous glucose monitoring in the self-management of type 2 diabetes: a paradigm shift. Diabetes Care 39, 71–73 (2016).
Allen, N. A., Fain, J. A., Braun, B. & Chipkin, S. R. Continuous glucose monitoring counseling improves physical activity behaviors of individuals with type 2 diabetes: a randomized clinical trial. Diabetes Res. Clin. Pract. 80, 371–379 (2008).
Yoo, H. J. et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res. Clin. Pract. 82, 73–79 (2008).
Ahn, Y. C. et al. Effectiveness of non-contact dietary coaching in adults with diabetes or prediabetes using a continuous glucose monitoring device: a randomized controlled trial. Health Care. 11, 252 (2023)
Mendes-Soares, H. et al. Assessment of a personalized approach to predicting postprandial glycemic responses to food among individuals without diabetes. JAMA Netw. Open 2, 2 (2019).
diabetes is expected to climb 51% to 700 million people by 2045. https://www.abbott.com/corpnewsroom/strategy-and-strength/cgm-leader-poised-for-more-growth.html
https://diabetes.org/about-diabetes/devices-technology/blood-sugar-checks-type-2
Kleinsinger F. The Unmet Challenge of Medication Nonadherence. Perm J. 2018;22:18-033. doi: 10.7812/TPP/18-033. PMID: 30005722; PMCID: PMC6045499. https://pubmed.ncbi.nlm.nih.gov/30005722/
https://www.globenewswire.com/en/news-release/2023/08/22/2729739/0/en/Continuous-Glucose-Monitoring-Devices-Market-Size-Share-to-Surpass-12-39-Billion-by-2030-Vantage-Market-Research.html
The lowdown on glycemic index and glycemic load - Harvard Health
Richardson KM, Jospe MR, Saleh AA, Clarke TN, Bedoya AR, Behrens N, Marano K, Cigan L, Liao Y, Scott ER, Guo JS, Aguinaga A, Schembre SM
Use of Biological Feedback as a Health Behavior Change Technique in Adults: Scoping Review J Med Internet Res 2023;25:e44359 doi: 10.2196/44359
Eiland L, Thangavelu T, Drincic A. Has Technology Improved Diabetes Management in Relation to Age, Gender, and Ethnicity? Curr Diab Rep. 2019 Nov 4;19(11):111. doi: 10.1007/s11892-019-1231-5. PMID: 31686221.
Sears et al 2022 P_CGM_Attitudes_and_Adoption_among_People_with_Type_2_Diabetes_Using_One_Drop https://www.researchgate.net/publication/361011857_705
https://diatribe.org/weightwatchers-and-cgm-new-trial-studies-using-both
Health Equity and Diabetes Technology: A Study of Access to Continuous Glucose Monitors by Payer, Geography and Race Executive Summary https://diabetes.org/sites/default/files/2023-09/ADA-CGM-Utilization-White-Paper-Oct-2022.pdf
Jospe 2024 https://www.frontiersin.org/articles/10.3389/fnut.2024.1301427
Wang JS, Xia PF, Ma MN, Li Y, Geng TT, Zhang YB, Tu ZZ, Jiang L, Zhou LR, Zhang BF, Tong WW, Shan Z, Liu G, Yang K, Pan A. Trends in the Prevalence of Metabolically Healthy Obesity Among US Adults, 1999-2018. JAMA Netw Open. 2023 Mar 1;6(3):e232145. doi: 10.1001/jamanetworkopen.2023.2145. PMID: 36892842; PMCID: PMC9999245.
, , , , , , , , Adding a Brief Continuous Glucose Monitoring Intervention to the National Diabetes Prevention Program: A Multimethod Feasibility Study, Journal of Diabetes Research, 2024, 7687694, 10 pages, 2024. https://doi.org/10.1155/2024/7687694
Hengist A, Ong JA, McNeel K, Guo J, Hall KD. Imprecision nutrition? Duplicate meals result in unreliable individual glycemic responses measured by continuous glucose monitors across four dietary patterns in adults without diabetes. medRxiv [Preprint]. 2023 Dec 11:2023.06.14.23291406. doi: 10.1101/2023.06.14.23291406. PMID: 37503002; PMCID: PMC10371100.
Richardson KM, Jospe MR, Bohlen LC, Crawshaw J, Saleh AA, Schembre SM. The efficacy of using continuous glucose monitoring as a behaviour change tool in populations with and without diabetes: a systematic review and meta-analysis of randomised controlled trials. Int J Behav Nutr Phys Act. 2024 Dec 23;21(1):145. doi: 10.1186/s12966-024-01692-6. PMID: 39716288; PMCID: PMC11668089.
https://www.news-medical.net/health/Why-Non-Diabetics-Are-Using-Continuous-Glucose-Monitors.aspx
